Professor Michael Sacks, a biomedical engineering faculty member at The University of Texas at Austin, has been awarded the 2026 ASME H.R. Lissner Medal in recognition of his “pioneering contributions to heart valve biomechanics utilizing highly innovative computer simulations.” His research bridges the cellular, tissue, and organ levels focused on restoration of normal function. The H.R. Lissner Medal is a prestigious society-level honor from the American Society of Mechanical Engineers (ASME), recognized as the highest award bestowed by ASME's Bioengineering Division.
As director of the Willerson Center for Cardiovascular Modeling and Simulation and principal faculty member at the Oden Institute for Computational Engineering and Sciences at UT, Sacks is an established pioneer in the mechanics of heart valves, with a focus on modelling disease mechanisms and developing rational simulation-based approaches for their functional restoration.
“This award is very important to me personally, as it recognizes nearly 15 years of concerted efforts at UT, and specifically at the Oden Institute's Willerson Center, in the study of deadly mitral valve and other heart valve diseases to develop new methods to improve treatment,” said Sacks.
Heart valve disease is projected to rise sharply with an aging population. Heart valves - structures within the heart that function like one-way gates—ensure that blood moves forward in the correct direction with each heartbeat. When those valves are compromised, the heart can become strained and lead to heart valve diseases.
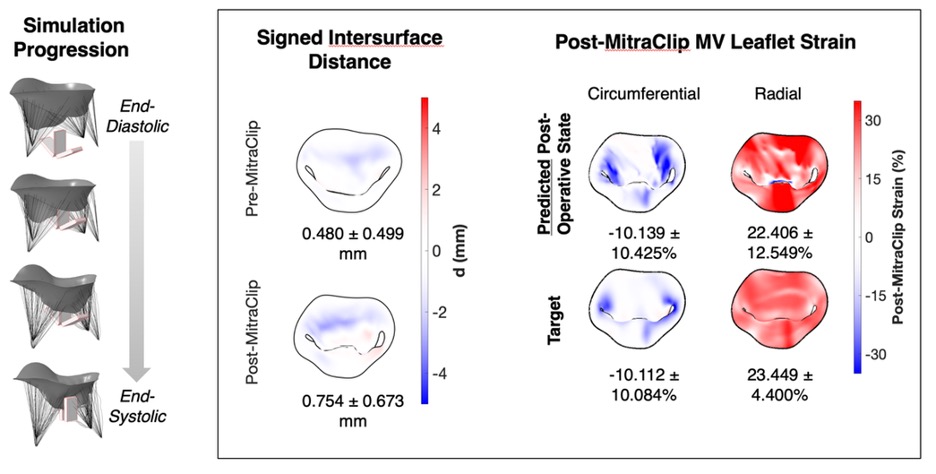
The primary focus for Sacks and his research team at the Willerson Center involves using an innovative approach to integrate multi-scale simulation methods—from cellular to organ level—to advance patient-specific computational models for mitral valve (MV) surgical repair to treat MV regurgitation—a result of tissue degeneration or myocardial infarction. While MV repair remains the preferred treatment, long-term outcomes remain subpar and difficult to predict.